Hydrogen - Online Test
Diborane is one of the following
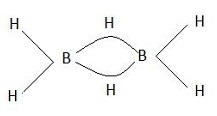
Diborane is said to be electron deficient because boron require 5 electrons to complete its octet. But in this molecule, each boron atom is bonded to 2 terminal hydrogen atoms each. Also, the two boron atoms are held together by two hydrogen atoms as shown below:
Metal hydrides (MHx) are the most technologically relevant class of hydrogen storage materials because they can be used in a range of applications including neutron moderation,electrochemical cycling, thermal storage, heat pumps, and purification/separation.
The metal hydrides such as intermetallic alloys and solid solutions have interstitial vacancies where atomic hydrogen is absorbed via an exothermic reaction; however, by endothermic path, the metal hydride desorbs the hydrogen reversibly at ambient to moderate temperatures.
