Hydrocarbons - Online Test
Q1. Which of the following will not show geometrical isomerism?
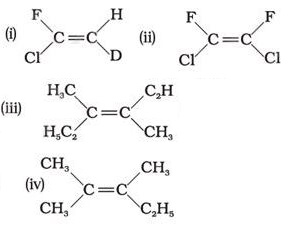

Answer : Option C
Explaination / Solution:
This is because geometrical isomerism is not possible if three groups are same.
Q2. Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
Answer : Option B
Explaination / Solution:
As we move from Cl to I,the atomic size increases,bond length increases,bond strength decreases,hence it becomes easier to release halogen and hence the order.I- is the a better leaving group.
Q3. Arrange the following carbanions in order of their decreasing stability.(A) H3C – C C– (B) H – C ≡ C– (C)
Answer : Option D
Explaination / Solution:
In B,the '-' charge is on sp hybridised C atom so the negative charge is stabilised.In A,there is an alkyl group attached to sp hybridised carbon which destabilises the negative charge. In C,the '-' charge is on a sp3 hybridised carbon and is hence the least stable.
Q4. Arrange the following alkyl halides in decreasing order of the rate of elimination reaction with alcoholic KOH
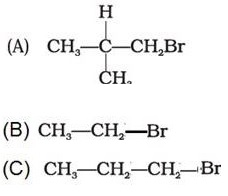

Answer : Option B
Explaination / Solution:
A more substituted alkene is preferred in accordance with the Saytzeff's rule.Hence the order.
Q5. Among the following compounds the one that is most reactive towards electrophilic nitration is
Answer : Option A
Explaination / Solution:
Methyl group is electron donating group, hence it increases the electron density in benzene ring thereby increasing the reactivity of the ring towards electrophilic substitution.
Q6. Which branched chain isomer of the hydrocarbon with molecular mass 72u gives only one isomer of mono substituted alkyl halide?
Answer : Option D
Explaination / Solution:
(CH3)3CCH3 + HX(X=Cl,Br,I) ------------> (CH3)3CCH2X
Hence a monosubstituted derivative is formed.
Q7. Ethyl benzene CANNOT be prepared by ______
Answer : Option C
Explaination / Solution:
Wurtz reaction is used to prepare alkanes with even number of carbon atoms.To prepare ethylbenzene,Wurtz Fittig reaction has to be used.
Q8. Identify B and D in the following sequence of reactions.


Answer : Option C
Explaination / Solution:
Ethanolis formed on addition of water, alcoholic KOH results in formation of alkene.
Q9. Ozonolysis of an organic compound 'A' produces an equimolar mixture of acetone and propionaldehyde .
Identify the organic compound 'A' .
Answer : Option A
Explaination / Solution:
2- Methyl -2 - pentene when treated with ozone forms an unstable ozonide intermediate . The ozonide when treated with yields an aldehyde (propionaldehyde ) and a ketone ( acetone ) as the final products The conversion reactions are represented as below.

Thus , the compound A is (2-Methyl -2 -pentene )
Q10. Ozonolysis of an organic compound gives formaldehyde as one of the products. This confirms the presence of
Answer : Option D
Explaination / Solution:
The presence of double bond on terminal carbon will give formaldehyde as one of the products.
