Alcohols Phenols and Ethers - Online Test
The rate of dehydration is related to the ease of formation of Carbocation and the energy of the carbocation intermediate. -.
Dehydration involves formation of carbocation 1st and since tertiary carbocation is more stable than secondary and primary therefore 3° is dehydrated first.
The ease of formation of Carbocation is Tertiary>Secondary>Primary.
The outcome of oxidation reactions of alcohols depends on the substituents on the carbinol carbon. In order for each oxidation step to occur, there must be H on the carbinol carbon.
- Primary alcohols can be oxidised to aldehydes or further to carboxylic acids. In aqueous media, the carboxylic acid is usually the major product. PCC or PDC, which are used in dichloromethane, allow the oxidation to be stopped at the intermediate aldehyde.
- Secondary alcohols can be oxidised to ketones but no further:
- Tertiary alcohols cannot be oxidised (no carbinol C-H)
 `
`

is known as
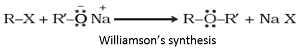
Williamson’s synthesis: When an alkyl halide reacts with sodium alkoxide, ether is formed. This reaction is known as Williamson’s synthesis. The reaction generally follows SN2 mechanism for primary alcohols.
Lucas Test is a test which is used to distinguish between primary, secondary and tertiary alcohols. This test is carried out with the help of Lucas reagent, which is a solution of anhydrous Zinc Chloride and concentrated hydrochloric acid (ZnCl2 + HCl). It is based on the difference between the reactivity of primary, secondary and tertiary alcohols with hydrogen halides.
