Alcohols Phenols and Ethers - Online Test
Carboxylic acids are reduced to primary alcohols in excellent yields by lithium aluminium hydride, a strong reducing agent.
However, LiAlH4 is an expensive reagent, and therefore, used for preparing special chemicals only. Commercially, acids are reduced to alcohols by converting them to the esters followed by their reduction using hydrogen in the presence of catalyst (catalytic hydrogenation).

- Aldehydes and ketones are most readily reduced with hydride reagents.
- The reducing agents LiAlH4 and NaBH4 act as a source of 4 x H- (hydride ion)
- Overall 2 H atoms are added across the C=O to give H-C-O-H
- Hydride reacts with the carbonyl group, C=O, in aldehydes or ketones to give alcohols.
- The substituents on the carbonyl dictate the nature of the product alcohol.
- Reduction of methanal (formaldehyde) gives methanol.
- Reduction of other aldehydes gives primary alcohols.
- Reduction of ketones gives secondary alcohols.
Fehling's Solution Test: When acetaldehyde is heated with Fehling solution, red ppt. of cuprous oxide are formed. But benzaldehyde does not give red ppt. with fehling solution.
Reaction are
CH3CHO + 2Cu2+ + 5OH-
CH3COO- + Cu2O + 3H2O
C6H5CHO + 2Cu2+ + 5OH-
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position.
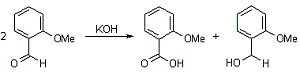
This redox disproportionation of non-enolizable aldehydes to carboxylic acids and alcohols is conducted in concentrated base.
IUPAC name of the following compound is

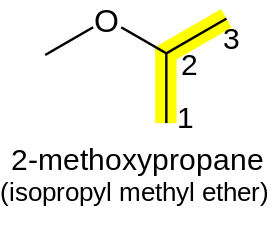
If the oxygen is not attached to the end of the main alkane chain, then the whole shorter alkyl-plus-ether group is treated as a side-chain and prefixed with its bonding position on the main chain. Thus CH3OCH(CH3)2 is 2-methoxypropane.
- The oxygen atom of an alcohol is sp3 hybridised and has two non bonding pairs of electrons.
- The O-H bond of alcohols is strongly polarised and hydrogen bonding occurs in much the same way as in water molecules
- As a consequence, alcohols have relatively high boiling points compared to other organic compounds of a similar molecular weight.
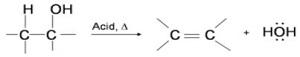
The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
The required range of reaction temperature decreases with increasing substitution of the hydroxy-containing carbon:
- 1° alcohols: 170° - 180°C
- 2° alcohols: 100°– 140 °C
- 3° alcohols: 25°– 80°C
If the reaction is not sufficiently heated, the alcohols do not dehydrate to form alkenes, but react with one another to form ethers.